Among all the infections which affect our bodies, those caused by fungi are the hardest to treat. They can affect the oral cavity, skin, nails, throat, lungs, and urinary tract among other parts of the body. One such well-known infection is candidiasis or candida which is characterized by the appearance of red spots on different parts of the skin resulting in pain and discomfort. It is caused by a fungus of the genus Candida and is very hard to treat. Despite the several antifungal options which are available to be recommended by the physicians, the patient may become resistant to those thus intensifying the condition.
Recently, a novel IV therapy called Rezafungin (Brand name Rezzayo) has been approved by the FDA for the cure of candidiasis. Let us take a look at what this newer drug is and how does it work?
Rezafungin-The newest treatment option
Rezafungin, sold under the brand name Rezzayo, is a long-acting anti-fungal drug that has recently been approved by the FDA. It is a novel treatment in the market for the treatment of candidiasis. Rezafungin comes in the form of an intravenous (IV) injection intended to be administered on a weekly basis.
How does it work?
The cell wall of Candida species is comprised of different chemical agents which include mannan, chitin, as well as a vast variety of glucans. These glucans make up almost 50 to 60% of the fungal cell wall which makes it one of the key components required for the integrity of a fungal cell.
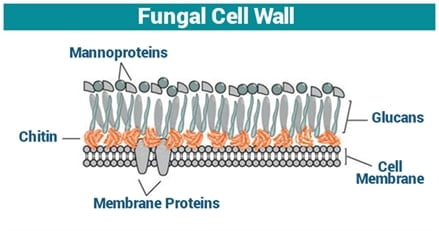
Glucans present in the cell wall of fungi which are targeted by Rezafungin and other echinocandin antifungal drugs
Rezafungin, just like the other members of the antifungal drug class echinocandin, works by inhibiting the synthesis of 1, 3-beta-D-glucan synthase, an enzyme necessary for the formation of these glucans. When glucans are not formed, the fungal cell wall is malformed resulting in the growth inhibition or death of a fungal cell due to the influx of fluids into it.
Dose and administration of Rezafungin
Rezzayo has been approved for the treatment of candidiasis in subjects equal to or older than 18 years of age who are not left with many treatment options other than Rezafungin. The recommended dose of lyophilized Rezzayo injection is 400 mg for the loading dose followed by a weekly administration of 200 mg Rezafungin. As the research data available on the duration of use of Rezafungin is only limited to 4 weeks, its use beyond that might cause side effects that need to be evaluated in further studies. While administration, the drug can be administered with 0.45% or 0.9% NaCl or dextrose 5% (D5W).
Applications of Rezafungin
Rezafungin is intended to be employed for the treatment of fungal infections which include:
- Invasive candidiasis caused by:
- Candida albicans
- Candida tropicalis
- Candida glabrata
- Candida parapsilosis
- Candidemia
Side effects of Rezafungin
When it comes to experiencing side effects with the use of Rezafungin, only less than 5% population has reported minor adverse reactions, if any. These include:
- Pyrexia i.e. fever
- Abdominal pain
- Anemia
- Vomiting
- Nausea
- Diarrhea
- Constipation
- Hypophosphatemia i.e. decreased phosphate levels
- Hypomagnesemia i.e. diminished levels of magnesium
- Hypokalemia i.e. lower levels of potassium
- Dysphagia i.e. difficulty in swallowing
- Gastrointestinal hemorrhage
- Insomnia
- Headache
- Dizziness
- Acute kidney injury
- Peripheral neuropathy
- Tremors
- Liver dysfunction
Limitations of Rezafungin injection
Being a new drug, Rezafungin still has a long way to go but before that, it has to be extensively tested in different scenarios to rule out every possibility of any severe side effects. In addition, some of the limitations of Rezzayo injection include the following:
- As specified by the FDA, Rezzayo injection has been tested on healthy subjects but not on individuals infected with fungal infections. It is a matter of concern as there may be other adverse drug reactions related to the therapy.
- Moreover, a lack ness exists in the establishment of data in terms of the side effects associated with the extended use of Rezafungin.
- The drug is also needed to be tested in patients with conditions such as osteomyelitis, endocarditis, and meningitis, among others.
- Data related to the efficacy as well as side effects of Rezafungin in pregnant and lactating women still needs to be established.
Contraindications of Rezafungin
If you have a history of hypersensitive reaction to any echinocandin or Rezafungin itself, the drug must be discontinued immediately. In case of a severe allergic reaction, medical help must be sought as soon as possible. Some of the symptoms which may appear in case of a hypersensitive or infusion-related reaction to the drug may include:
- Nausea
- Sensation of warmth
- Flushing
- Urticaria
- Chest tightness
- Photosensitivity
Furthermore, Rezafungin has been found to alter the liver-related parameters so closer monitoring is needed in this respect.
Rezafungin vs Caspofungin
During the trials, both the antifungal drugs Rezafungin and Caspofungin were compared to evaluate if one of them dominates the effects of the other. However, it was found that Rezafungin, the weekly injectable is ‘not inferior to’ the daily-injected Caspofungin suggesting that both drugs provide similar beneficial effects to the patients during treatment.
Conclusion
Among all the latest advancements in the field of medicine, Rezafungin intravenous injection is a newer addition. It has been recently given FDA approval which means its marketing can be started as early as the summer of 2023. The drug is in power with the drug Caspofungin which is already in use for the treatment of invasive candidiasis and other fungal infections. Although the research data available on Rezafungin is limited, further studies can pave the way for its extensive use in the future if no serious side effects are found to be associated with it.

PhD Scholar (Pharmaceutics), MPhil (Pharmaceutics), Pharm D, B. Sc.
Uzma Zafar is a dedicated and highly motivated pharmaceutical professional currently pursuing her PhD in Pharmaceutics at the Punjab University College of Pharmacy, University of the Punjab. With a comprehensive academic and research background, Uzma has consistently excelled in her studies, securing first division throughout her educational journey.
Uzma’s passion for the pharmaceutical field is evident from her active engagement during her Doctor of Pharmacy (Pharm.D) program, where she not only mastered industrial techniques and clinical case studies but also delved into marketing strategies and management skills.
Throughout her career, Uzma has actively contributed to the pharmaceutical sciences, with specific research on suspension formulation and Hepatitis C risk factors and side effects. Additionally, Uzma has lent her expertise to review and fact-check articles for the Health Supply 770 blog, ensuring the accuracy and reliability of the information presented.
As she continues her PhD, expected to complete in 2025, Uzma is eager to contribute further to the field by combining her deep knowledge of pharmaceutics with real-world applications to meet global professional standards and challenges.