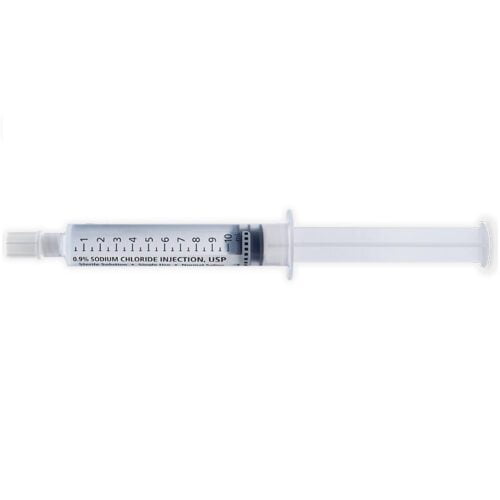
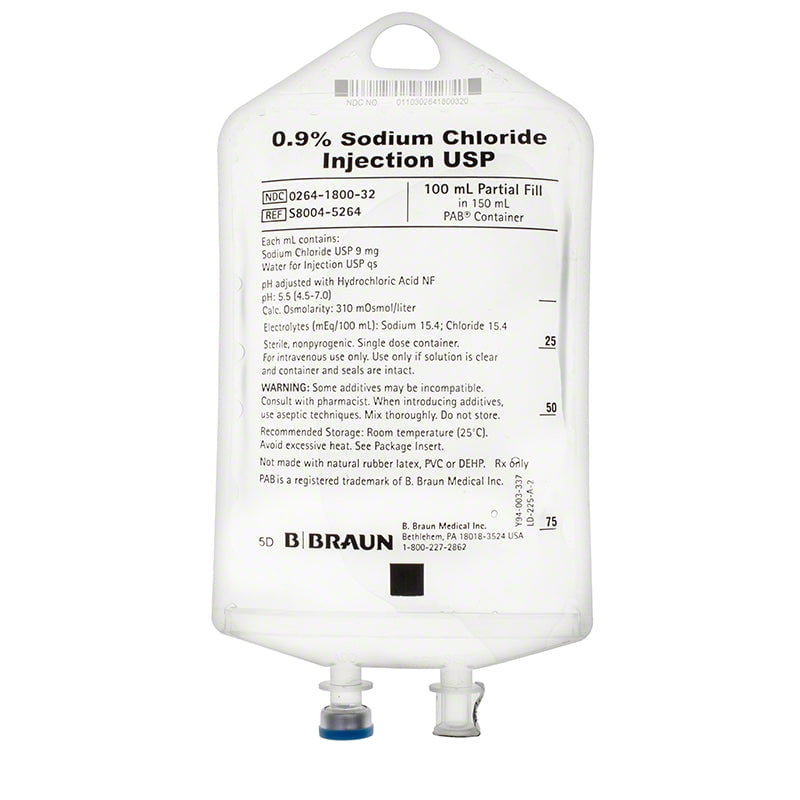
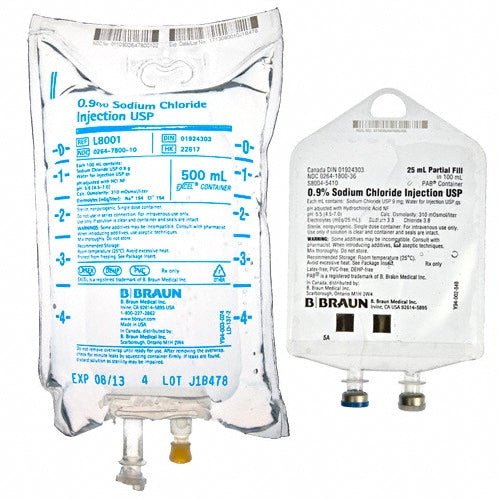
At Health Supply 770 Inc. speed is key. We take great pride in our ability to quickly prepare quotes, ship, and deliver high-quality medical equipment . Which makes us the ideal destination for all your medical needs.

Customer Service: 770-874-0431
[email protected]
7111 Bayou George Dr. Panama City
Florida 32404