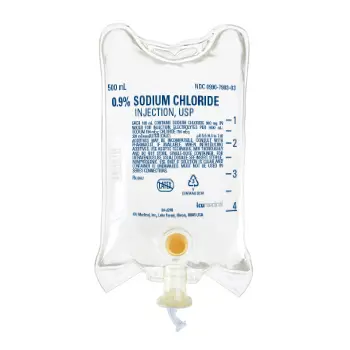
ICU Hospira Sodium Chloride 0.9% Injection IV Solution 500 mL 24/Cs
Description
This solution is intended exclusively for parenteral use and is formulated for the dilution or dissolution of drugs requiring an aqueous vehicle prior to injection.
Composition
0.9% Sodium Chloride Injection, USP is a sterile, nonpyrogenic, isotonic solution of sodium chloride in water. Each mL contains 9 mg of sodium chloride. The solution is preservative-free, containing no bacteriostatic or antimicrobial agents, and is supplied only in single-dose containers for drug dilution or dissolution. Its osmolality is approximately 0.308 mOsmol/mL (calculated). Hydrochloric acid and/or sodium hydroxide may be used for pH adjustment, resulting in a pH of 5.3 (range: 4.5–7.0).
Chemical Properties
Sodium Chloride, USP is chemically designated as NaCl, a white crystalline compound that is highly soluble in water.
Additional Information
- Supplied in 500 mL containers, conveniently packaged as 24 bags per case.
- Suitable for use in intensive care units (ICU) and other medical settings requiring sterile IV solutions.
















Reviews
Clear filtersThere are no reviews yet.