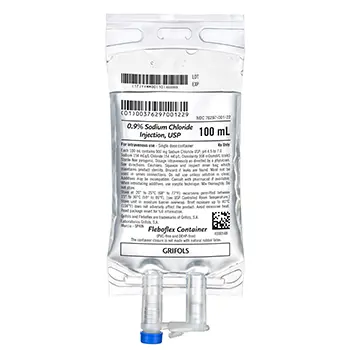
The ICU Medical 731358 0.9% Sodium Chloride Injection, USP, is a sterile, non-pyrogenic solution intended for intravenous administration. Each 100 mL contains 900 mg of Sodium Chloride, providing 154 mEq/L of sodium and 154 mEq/L of chloride, with an osmolarity of 308 mOsmol/L. The solution is packaged in a 100 mL flexible bag fabricated from non-PVC and non-DEHP materials, ensuring compatibility and safety during administration.
Packaging Details: Each case contains 70 bags of the 100 mL solution.
Key Features:
- Sterile and Non-Pyrogenic: Ensures patient safety during intravenous administration.
- Non-PVC/Non-DEHP Bag: Reduces the risk of plasticizer-related complications and enhances drug compatibility.
- Isotonic Solution: Matches the body’s natural fluid balance, making it safe for intravenous use.
- Flexible and Lightweight: Facilitates easy handling and storage.
- Quantity per Case: 70 bags per case, suitable for healthcare facilities.
- National Drug Code (NDC): 76297-001-21
Intended Use: This solution is commonly used for intravenous fluid and electrolyte replenishment and as a priming solution in hemodialysis procedures. It should be administered under the guidance of a healthcare professional.
Storage Instructions: Store in a cool, dry place away from direct sunlight. Ensure the integrity of the container before use.
Note: This product is intended for intravenous use only and should be administered by qualified healthcare personnel. Always check for compatibility and patient-specific factors before administration.















Reviews
Clear filtersThere are no reviews yet.