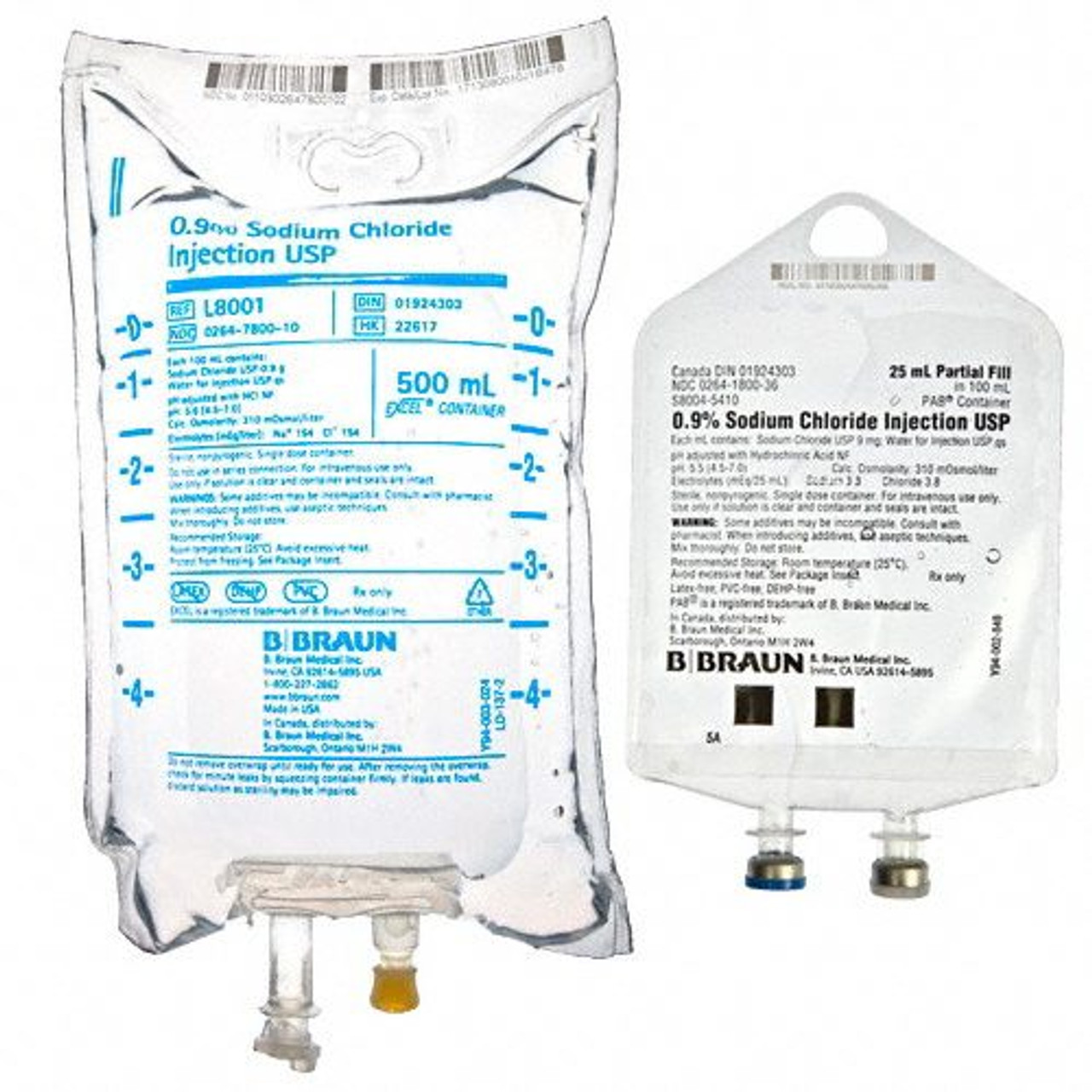
B. Braun Q8001 0.9% Sodium Chloride Injection, USP, 500 mL 24/Cs
$9.99
Payment Methods:

Description
The B. Braun Q8001 0.9% Sodium Chloride Injection, USP, is a sterile, non-pyrogenic, isotonic solution intended for intravenous administration. Each 100 mL contains 900 mg of Sodium Chloride, providing 154 mEq/L of sodium and 154 mEq/L of chloride, with an osmolarity of 308 mOsmol/L. The solution is packaged in a 500 mL EXCEL® Plus IV container, which is free from PVC and DEHP, reducing the risk of plasticizer-related complications.
Packaging Details: Each case contains 24 bags of the 500 mL solution.
Key Features:
- Sterile and Non-Pyrogenic: Ensures patient safety during intravenous administration.
- PVC-Free and DEHP-Free Container: Reduces the risk of plasticizer-related complications and enhances drug compatibility.
- Isotonic Solution: Matches the body’s natural fluid balance, making it safe for intravenous use.
- Flexible and Lightweight: Facilitates easy handling and storage.
- Quantity per Case: 24 bags per case, suitable for healthcare facilities.
Intended Use: This solution is commonly used for intravenous fluid and electrolyte replenishment, treatment of metabolic alkalosis in the presence of fluid loss, and mild sodium depletion. It should be administered under the guidance of a healthcare professional.
Storage Instructions: Store in a cool, dry place away from direct sunlight. Ensure the integrity of the container before use.
Note: This product is intended for intravenous use only and should be administered by qualified healthcare personnel. Always check for compatibility and patient-specific factors before administration.
Shop with confidence
B. Braun Q8001 A sterile, non-pyrogenic 0.9% Sodium Chloride intravenous solution in a 500 mL EXCEL® Plus IV container, used for fluid and electrolyte replenishment.
Customer Reviews
You must be logged in to post a review.
Related products
ICU Medical Hospira 5% Dextrose and 0,45% Sodium Chloride Injection 1000 mL 12/Cs
Available on backorder
















Reviews
Clear filtersThere are no reviews yet.