Back to products


Baxter 2B0043 0.9% Sodium Chloride Injection USP 100 mL - Expires January 2025
$399.88 Original price was: $399.88.$79.88Current price is: $79.88.
Baxter 2B0042 0.9% Sodium Chloride Injection, USP – 50 mL
SKU:
Baxter 2B0042
34
People watching this product now!
Payment Methods:

Description
Title: Baxter 2B0042 0.9% Sodium Chloride Injection, USP – 50 mL MINI-BAG Plus Container
Product Description:
The Baxter 2B0042 – 0.9% Sodium Chloride Injection, USP is a sterile, nonpyrogenic intravenous solution designed for reconstitution and delivery of powdered or liquid drugs in a safe, controlled manner. Packaged in a 50 mL MINI-BAG Plus Container, it is an essential diluent for drug admixture and electrolyte replenishment.
Key Features:
- Strength: 0.9% Sodium Chloride
- Volume: 50 mL
- Container Type: MINI-BAG Plus with an integral drug vial adaptor for secure and sterile drug admixture.
- Sterile and Nonpyrogenic: Suitable for intravenous administration with no antimicrobial agents.
- Osmolarity: 308 mOsmol/L
- Composition: 900 mg Sodium Chloride per 100 mL, 154 mEq/L Sodium, and 154 mEq/L Chloride.
- Material: VIAFLEX Plastic Container (PL 146), minimizing risk of leaching.
Indications and Uses:
- A source of water and electrolytes.
- Diluent for reconstitution of single-dose powdered or liquid drug vials (up to 10 mL) with 20 mm closures.
- Supports hydration and fluid balance in patients.
Precautions:
- Monitor patients for signs of hypersensitivity or electrolyte imbalances.
- Use cautiously in cases of fluid overload, hyponatremia, hypernatremia, or severe renal impairment.
- Adverse reactions may include infusion site reactions, metabolic imbalances, or hypersensitivity.
Specifications:
- Manufacturer: Baxter
- Manufacturer #: 2B0042
- Generic Drug Name: Sodium Chloride
- NDC Number: 00338055311
- Country of Origin: United States
- Packaging: Quad Pack, 80 units per case
Shop with confidence
Terms And Conditions |
Refund Policy
×
Specifications: Baxter 2B0042
- Manufacturer: Baxter
- Manufacturer #: 2B0042
- Generic Drug Name: Sodium Chloride
- NDC Number: 00338055311
- Country of Origin: United States
- Packaging: Quad Pack, 80 units per case
Customer Reviews
Rated 0 out of 5
0 reviews
Rated 5 out of 5
0
Rated 4 out of 5
0
Rated 3 out of 5
0
Rated 2 out of 5
0
Rated 1 out of 5
0
Be the first to review “Baxter 2B0042 0.9% Sodium Chloride Injection, USP – 50 mL” Cancel reply
You must be logged in to post a review.
Related products
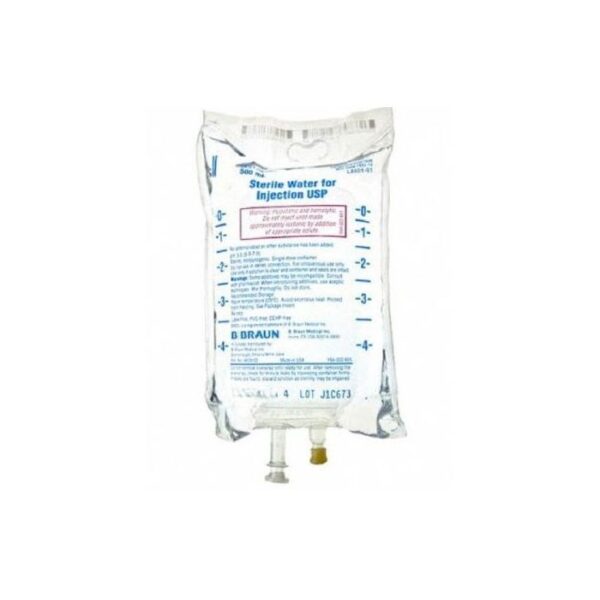
B Braun L8501-01 Sterile Water for Injection USP 500 mL 24/CS
Rated 0 out of 5
In stock
SKU:
L8501-01
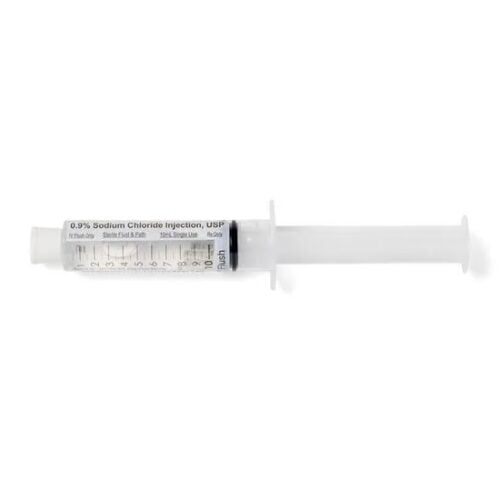
Medline EMZE010001 IV Flush Solution Sodium Chloride, Preservative Free 0.9% Injection Prefilled Syringe 10 mL 600/CS
Rated 0 out of 5
In stock
$15.88 – $741.88Price range: $15.88 through $741.88
Select options
This product has multiple variants. The options may be chosen on the product page
















Reviews
Clear filtersThere are no reviews yet.