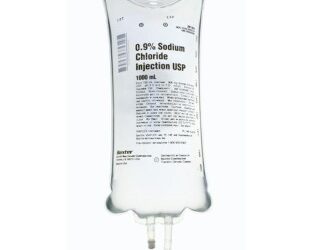
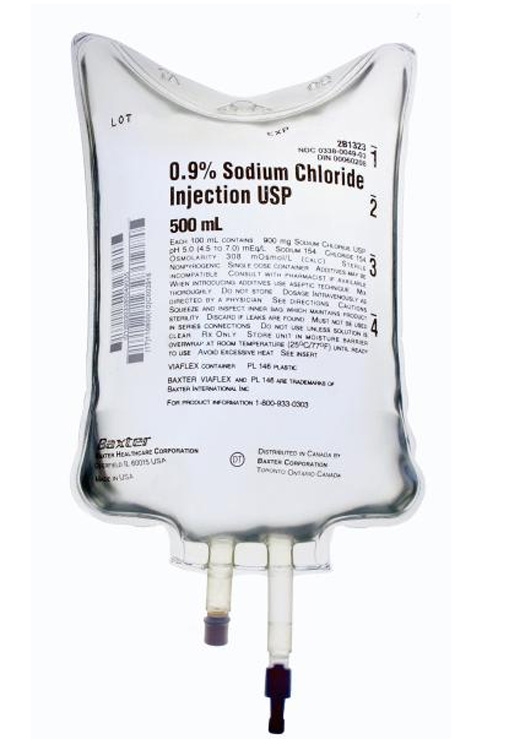
Baxter 2B1323Q Sodium Chloride Injection, USP 0.9% – 500 mL 24/CS
$379.88 Original price was: $379.88.$149.88Current price is: $149.88.
18 in stock
18 in stock
Payment Methods:

Description
Baxter 2B1323Q Sodium Chloride Injection, USP 0.9% is a sterile, nonpyrogenic solution designed for intravenous fluid and electrolyte replenishment. Packaged in a flexible VIAFLEX® IV bag, this preservative-free solution is ideal for single-dose administration in medical settings.
Key Features:
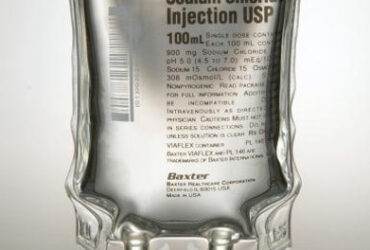
- Composition: Contains 9 g/L Sodium Chloride (NaCl) with an osmolarity of 308 mOsmol/L.
- Sterile and Preservative-Free: Ensures safety and efficacy for intravenous use.
- pH Range: 5.0 (4.5 to 7.0).
- Container: VIAFLEX® bag crafted from PL 146 Plastic, a specially formulated polyvinyl chloride material.
- Latex-Free: Not made with natural rubber latex.
Baxter 2B1323Q Specifications:
- Volume: 500 mL.
- Strength: 0.9%.
- Container Type: Flexible VIAFLEX® IV Bag.
- Manufacturer: Baxter (Manufacturer #: 2B1323Q).
- Country of Origin: United States.
- NDC Number: 00338-0049-03.
- UNSPSC Code: 51191602.
Important Notes:
This solution may interact with the plastic container, leading to trace amounts of chemical leaching (e.g., DEHP, up to 5 ppm) within the expiration period.
Sodium Chloride Injection, USP 0.9% in a 500 mL VIAFLEX® IV bag is a trusted choice for effective hydration and electrolyte therapy in clinical environments. For more details or to place an order, contact your representative.
Shop with confidence
Baxter 2B1323Q Specifications:
- Volume: 500 mL.
- Strength: 0.9%.
- Container Type: Flexible VIAFLEX® IV Bag.
- Manufacturer: Baxter (Manufacturer #: 2B1323Q).
- Country of Origin: United States.
- NDC Number: 00338-0049-03.
- Product Dating: Ships with at least 60 days remaining shelf life.
- UNSPSC Code: 51191602.
Related products
ICU Medical Hospira Sodium Chloride 0.9% Injection IV Solution 250 mL 24/Cs
Out of stock
B. Braun L8500 Sterile Water for Injection USP 1000 mL
In stock
Customer Reviews
You must be logged in to post a review.















Reviews
Clear filtersThere are no reviews yet.