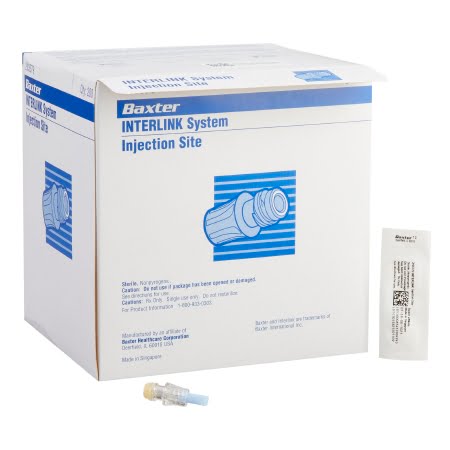
Baxter 2N3379 Interlink Luer Lock Injection Site – Negative Displacement
SKU:
2N3379
$818.88
32
People watching this product now!
Payment Methods:

Description
Baxter 2N3379 Interlink Luer Lock Injection Site – Negative Displacement
The Baxter 2N3379 is a needleless connector, specifically an Interlink Luer Lock injection site, designed for use with Interlink systems. It features a negative displacement design.
Key Features:
- Manufacturer: Baxter.
- Product Code: 2N3379.
- Brand: Interlink®.
- Type: Needleless connector.
- Application: Injection site for Interlink systems.
- Connection: Luer lock.
- Displacement: Negative displacement.
- NDC Number: 85412004940.
- UNSPSC Code: 42221602.
- Latex-Free: Not made with natural rubber latex.
- Packaging: 800 units per case.
Intended Use:
- To provide a needleless access point for intravenous medication or fluid administration within Interlink systems.
- To reduce the risk of needlestick injuries.
Benefits:
- Secure connections: Luer lock compatibility ensures tight, leak-proof connections.
- Reduced risk of contamination: Single-use design and self-sealing membrane minimize infection risks.
- Enhanced patient comfort: Rotatable design may allow for better positioning and reduce irritation.
- Streamlined workflow: Disposable design simplifies procedures and reduces the need for cleaning and sterilization.
- Cost-effective: Bulk packaging can be economical for high-volume use.
- Negative displacement: Helps prevent backflow and potential catheter occlusion.
- Latex Free: Reduces the risk of allergic reaction.
Shop with confidence
Terms And Conditions |
Refund Policy
×
The Baxter 2N3379 is a negative displacement, luer lock injection site, designed for needleless intravenous access, emphasizing safety and efficiency within Interlink systems.
Customer Reviews
Rated 0 out of 5
0 reviews
Rated 5 out of 5
0
Rated 4 out of 5
0
Rated 3 out of 5
0
Rated 2 out of 5
0
Rated 1 out of 5
0
Be the first to review “Baxter 2N3379 Interlink Luer Lock Injection Site – Negative Displacement” Cancel reply
You must be logged in to post a review.
Related products
BD 381137 Angiocath IV Catheters 20 Gauge 1.88 Inch Without Safety CS/4
Rated 0 out of 5
In stock
$332.88



















Reviews
Clear filtersThere are no reviews yet.