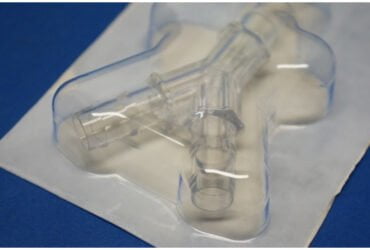
BD 303346 Syringe 3cc 17 Gauge Interlink System IV Blunt Cannula w/oNdl 800/CS
$364.88
Payment Methods:

Description
BD 303346 Syringe 3cc 17 Gauge Interlink System IV Blunt Cannula w/oNdl 800/CS

Syringes with BD Cannula for use with Baxter Interlink, Abbott LifeShield, or B. Braun SafeLine Systems
303346 – 3 mL Syringe with BD Blunt Plastic Cannula (syringe cannula)
The BD blunt plastic cannula is a truly universal cannula designed to be used with all types of split septum IV injection sites and vials for needleless access.
Compatibility
The cannula and accessories are compatible with BD VersaSafe, Abbott LifeShield, B. Braun Safeline and Baxter Interlink IV access systems.

Key Product Features
| Sterile | Sterilized product |
| Safety Engineered | Safety engineered product |
| Safety Engineered Feature | Needleless |
| Sterilization Method | Gamma irradiation |
| BPA Free | Not made with BPA |
| DEHP Free | Not made with DEHP |
| Latex Statement | Not made with natural rubber latex |
| PVC Free | Not made with PVC |
| Disposable | Disposable product |
| Single Use | Product is for single use only |
Regulatory Compliance and Quality System
BD Products comply with the regulatory requirements of the region in which these are sold and manufactured.
Sterility
All products which are labeled as #sterile# and released for sale by BD are certified to be sterile as long as the package is unopened and undamaged. For those products labeled #sterile fluid path#, only the fluid path is sterile.
This product is primarily sterilized via E-beam. Sterilization cycle development/validation is performed to 10-6 SAL in accordance with current ISO 11137 guidelines.
Pyrogenicity
All products which are labeled as non-pyrogenic and released for sale by BD have been tested per United States Pharmacopeia (USP) chapter 85 – Bacterial Endotoxins Test and meets limits as specified in chapter 161- Transfusion and Infusion Assemblies and Similar Medical Devices.
Biocompatability
This product has been evaluated in accordance with ISO 10993 “Biological Evaluation of Medical Devices”, and complies with all relevant sections.
Quality Control Testing and Release
Representative production samples are collected and inspected in accordance with current applicable product specifications. Inspection records are reviewed and signed off by qualified personnel for product release. The released devices meet applicable BD product specifications.
Device Characteristics
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437) | No |
| Device labeled as “Not made with natural rubber latex” | No |
| For Single-Use | Yes |
| Prescription Use (Rx) | Yes |
| Over the Counter (OTC) | No |
| Kit | No |
| Combination Product | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P) | No |
Product Packaging Information
| Packaging Level | Shelfpack | Each | Case |
| Quantity | 100 | 1 | 800 |
| Length | 210.0 mm | 419.0 mm | |
| Width | 188 mm | 381.0 mm | |
| Height | 170 mm | 330.0 mm | |
| Weight | 620.0 g | 5.2 g | 5.375 kg |
Shop with confidence
Related products
BD 305916 Hypodermic Needle SafetyGlide Sliding Safety Needle 25 Gauge 1 Inch Length 500/CS
In stock
BD 930825 Prep Solution Chloraprep Scrub Teal 26 Ml Applicator 2% / 70% Strength Chg Isopropyl Alcohol Sterile 25/CS
In stock
BD 305210 Oral Medication Syringe 3 mL Bulk Pack Luer Slip 500/CS
In stock