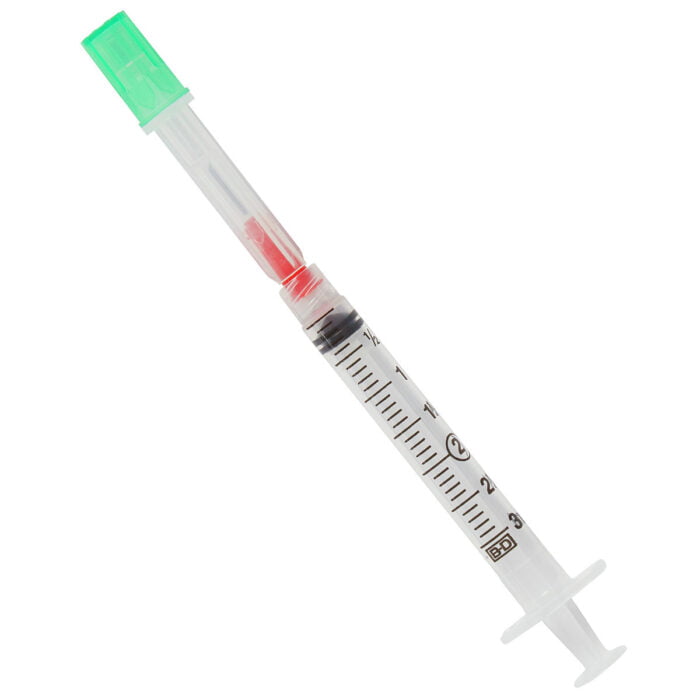
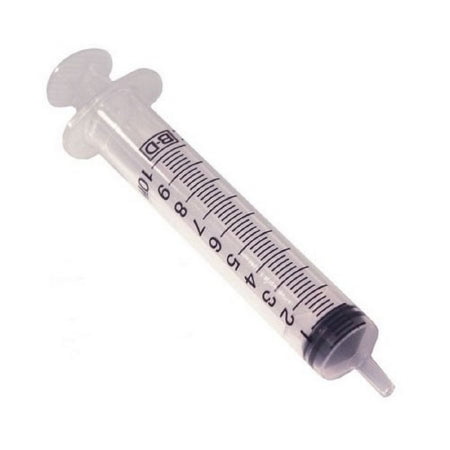
BD 303392 Syringe 5cc Interlink w/ 20 Gauge Cannula Twin Pack 400/CS
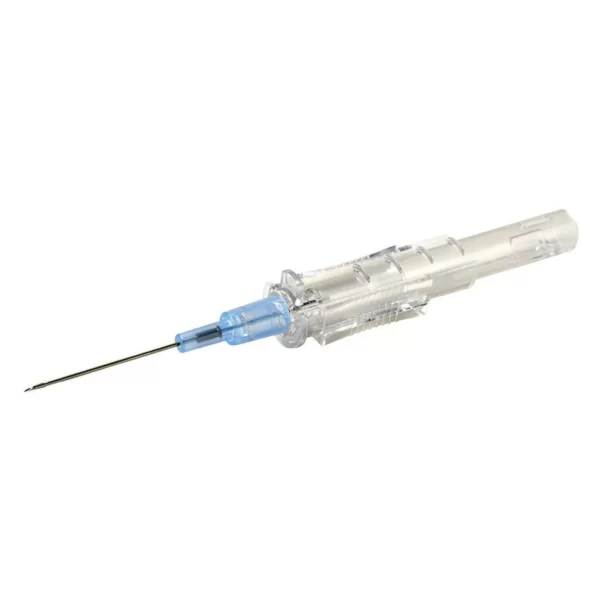
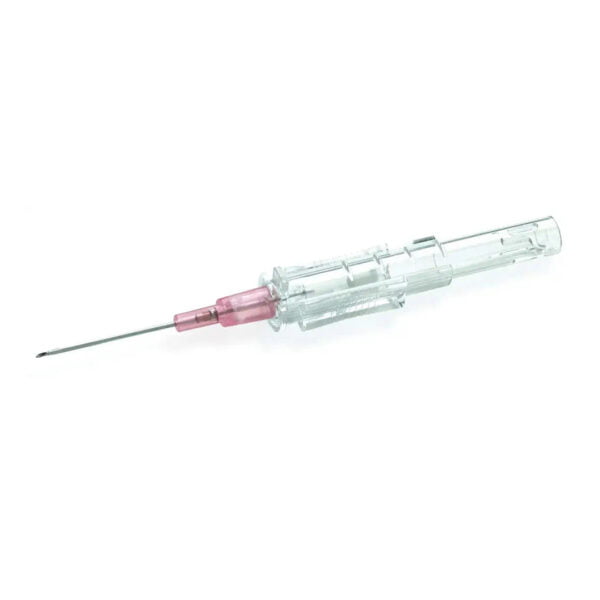
303392 – 5 mL Syringe with BD Twinpak Dual Cannula Device
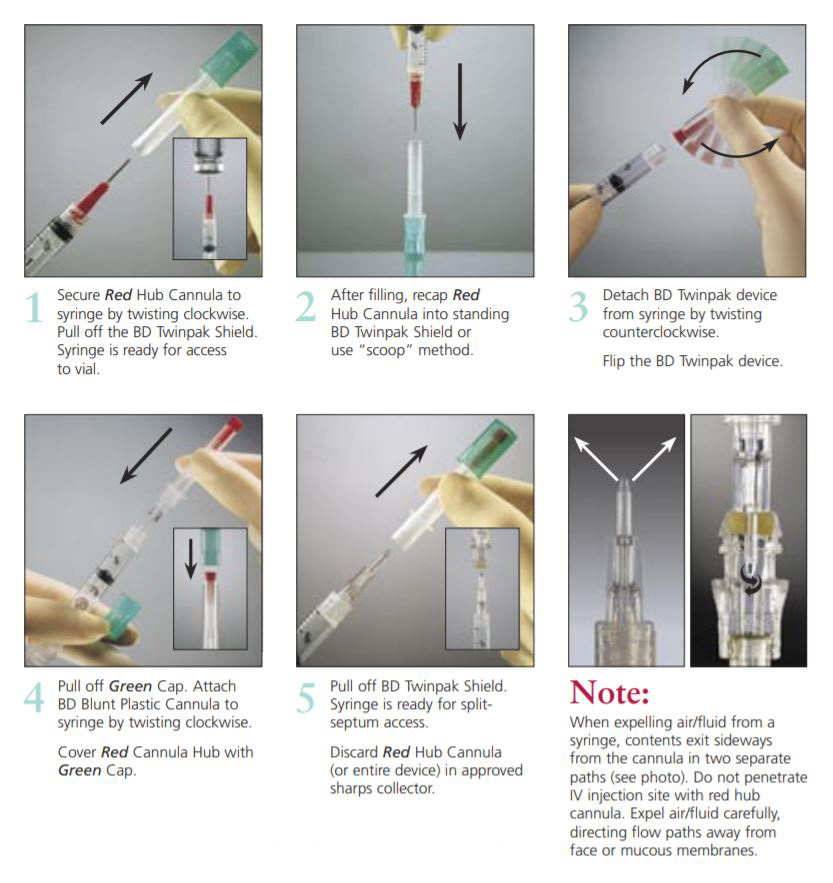
The BD Twinpak Dual Cannula is a truly universal device that simplifies needleless IV access. It offers twice the performance of traditional methods. BD Twinpak Dual Cannula features two cannulas in one shield: one red-hub, steel cannula for syringe filling and one clear, blunt plastic cannula for safe access into a split septum. The BD Twinpak Device is designed to help streamline IV work flow and reduce the number of individual pieces, thereby saving time and reducing costs. For use with Interlink, Abbott LifeShield or SafeLine Systems.
BD Twinpak Dual Cannula Device
The Self-Contained Solution

Red Hub Cannula (Syringe Filling Device) |
Blunt Plastic Cannula (with Green Cap) |
| 20 G Metal Cannula
provides versatility in syringe filling from vials, and for other multiple vial access needs (e.g., reconstitution).Blunt Tip Design provides a safer alternative to a traditional, sharp needle for syringe filling.Red Hub easily identifies the syringe filling device. |
Dual Side Ports
provide greater fluid dispersion in flushing and drug deliveryCenter-point Design features tapered tip for less resistance during insertionUniversally Compatible with split-septum cannula access devices, such as Baxter Interlink, Hospira LifeShield, B. Braun SafeLine, and Alaris VersaSafe injection sites as well as vials designed for needleless access.Green Cap designed to provide easy identification and a protective cover for clear BD Blunt Plastic Cannula Hub, and can later be used to cover the BD Red Hub Cannula. |
Key Product Features
| Sterile | Sterilized product |
| Safety Engineered | Safety engineered product |
| Safety Engineered Feature | Needleless |
| Sterilization Method | Gamma irradiation |
| BPA Free | Not made with BPA |
| DEHP Free | Not made with DEHP |
| PVC Free | Not made with PVC |
| Latex Statement | Not made with natural rubber latex |
| Disposable | Disposable product |
| Single Use | Product is for single use only |
Device Characteristics
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437) | No |
| Device labeled as “Not made with natural rubber latex” | No |
| For Single-Use | Yes |
| Prescription Use (Rx) | Yes |
| Over the Counter (OTC) | No |
| Kit | No |
| Combination Product | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P) | No |
Instructions for Use
Product Packaging Information
| Packaging Level | Shelfpack | Each | Case |
| Quantity | 100 | 1 | 400 |
| Length | 27.31 cm | 273.0 mm | |
| Width | 20.0 cm | 400.0 mm | |
| Height | 19.69cm | 393.7 mm | |
| Weight | 1.18 kg | 11.8 g | 4.71 kg |












Reviews
Clear filtersThere are no reviews yet.