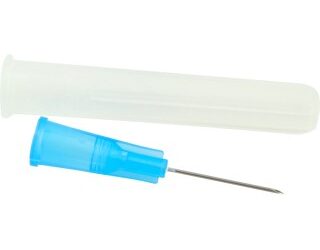
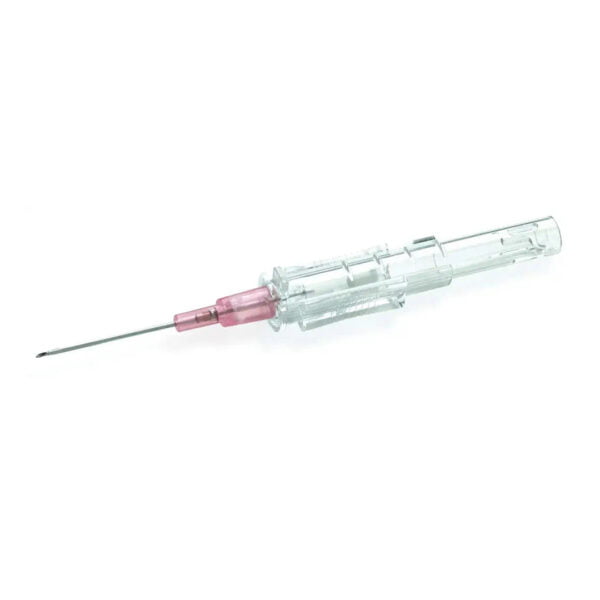
BD 305180 Needle 18 Gauge 1-1/2 Inch Blunt 1000/CS
$122.88
Payment Methods:

Description
BD 305180 Needle 18 Gauge 1-1/2 Inch Blunt 1000/CS
BD 305180 Needle 18 Gauge 1-1/2 Inch Blunt 1000/CS
305180 – 18 G BD Blunt Fill Needle 1 1/2 in. Single use, Sterile
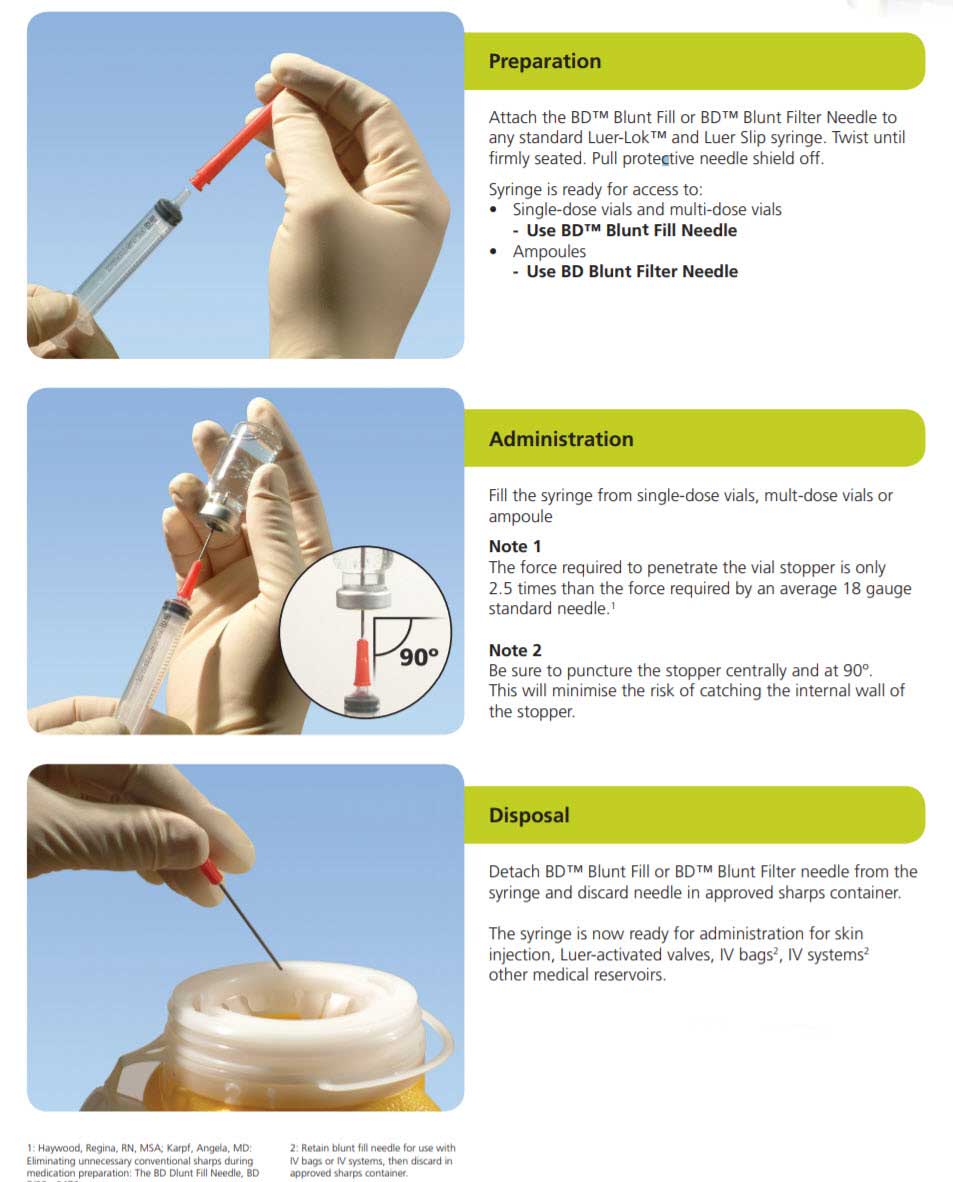
As a healthcare institution, your objectives are simple: remove conventional needles; provide safety-engineered alternatives; and manage costs. The BD Blunt Needle family allows you to meet these objectives:
Features
|
Benefits
|
Key Product Features
| CE Mark | Product is CE-marked |
| Hub Color | Red |
| Hub Material | Polypropylene |
| Hub Type | Luer |
| Needle Gauge | 18 G |
| Needle Gauge (m) | 1.20 mm |
| Needle Length (in.) | 1 1/2 in. |
| Needle Length (m) | 38.10 mm |
| Needle Tip Type | Blunt |
| Needle Type | Fill |
| Needle Wall Type | Regular |
| Total Shelf Life | 1825 |
| Sterile | Sterilized product |
| Type Of Bevel | Blunt |
| Sterilization Method | Radiation |
| BPA Free | Not made with BPA |
| DEHP Free | Not made with DEHP |
| PVC Free | Not made with PVC |
| Latex Statement | Not made with natural rubber latex |
| Disposable | Disposable product |
| Single Use | Product is for single use only |
Types of Needle Walls and Bevels
The Thin Wall needle is thinner than a Regular Wall needle. The inner diameter is bigger without any increase in the outside diameter. For example, a 21 G Thin Wall needle has the same outside diameter as a 21 G Regular Wall, but has the same inner diameter as a 20 G needle. Thin Wall allows the same flow volume as a Regular Wall needle one gauge larger.
305181 — Regular Wall
This is the most common wall thickness. The thickness of the steel wall allows a good flow rate, and minimizes flexing when the needle is inserted into a vial stopper or patient.
305181 — Blunt bevel
Blunt bevel:The blunt tip is used for injection into soft tissue and when a uniform solution distribution is needed. The beveled style is used for applications that involve the penetration of a tough tissue. A blunt fill needle is designed to reduce the potential for needlestick injuries.
Stainless Steel Needle
Made of stainless steel, which is siliconized to allow easier penetration, thus minimizing patient discomfort. Available in different lengths and gauges to suit individual clinical and patient needs.
Device Characteristics
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437) | No |
| Device labeled as “Not made with natural rubber latex” | No |
| For Single-Use | Yes |
| Prescription Use (Rx) | Yes |
| Over the Counter (OTC) | No |
| Kit | No |
| Combination Product | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P) | No |
Product Packaging Information
| Packaging Level | Shelfpack | Each | Case |
| Quantity | 100 | 1 | 1000 |
| Length | 116.0 mm | 451.0 mm | |
| Width | 88.0 mm | 206.0 mm | |
| Height | 98.0 mm | 129.0 mm | |
| Weight | 152.5 g | 1.525 g | 1.525 kg |
Recommended Points to Practise
Shop with confidence
BD 305180 Needle 18 Gauge 1-1/2 Inch Blunt 1000/CS
Related products
Retractable Technologies 24131 Blood Collection Set VanishPoint® 21 Gauge 3/4 Inch Needle Length Safety Needle 12 Inch Tubing Sterile
In stock
BD 305210 Oral Medication Syringe 3 mL Bulk Pack Luer Slip 500/CS
In stock
SMITHS MEDICAL PROTECTIVE SAFETY I.V. CATHETER 22G X 1″ 3050 Box of 50
Out of stock
Customer Reviews
You must be logged in to post a review.


















Reviews
Clear filtersThere are no reviews yet.