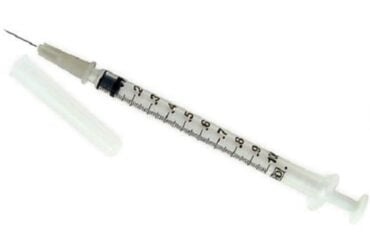
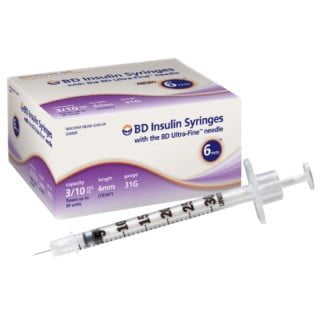
BD 324909 Syringe Insulin 3/10ML 31 Gauge 15/64 Inch 500/CS
$351.50
Payment Methods:

Description
BD 324909 Syringe Insulin 3/10ML 31 Gauge 15/64 Inch 500/CS

324909 – BD Veo Insulin Syringes with the BD Ultra-Fine needle 6mm x 31G 3/10 mL/cc
BD Veo insulin syringes with BD Ultra-Fine 6mm x 31G needle
BD Veo insulin syringes feature the shortest BD Ultra-Fine needle, 53% shorter than the 12.7-mm needle. This length is supported by recent recommendations published in Mayo Clinic Proceedings that advocate using the shortest needle first-line for all patient categories. Preferred by most patients over their current needle length, BD Veo insulin syringes provide approximately 8x-lower risk of intramuscular injection compared to a 12.7-mm needle.
- Greater Efficacy: Efficacy equivalent to longer needles
- Patient Choice: Preffered by over 80% patients
- Less intimidating: Less intimidating for patients
- Reduced Risk: Reduces the risk of intramuscular injection
- Self-contained insulin syringe with permanent needle for use with U-100 insulin.
- Packaging contains end user directions and information.
Reduced risk of hypoglycemia
Longer needle lengths may lead to frequent IM vs subcutaneous tissue injections, which may cause unexplained hypoglycemia.
Introducing the BD Insulin Syringe with the BD Ultra-Fine 6mm Needle
Over 80% of patients with diabetes preferred the BD Insulin Syringe with the NEW BD Ultra-Fine 6mm needle to their current needle1,2
- 1. Compared with the 12.7mm x 30G.
- 2. Current needle sizes: 12.7mm and 8mm.

Key Product Features
| Hub Color | Clear |
| Hub Material | Polypropylene |
| Hub Type | Rounded |
| Needle Gauge | 31 G |
| Needle Gauge (m) | 0.25 mm |
| Needle Length (in.) | 1/4 in. |
| Needle Length (m) | 6 mm |
| Needle Tip Type | 3-bevel |
| Needle Type | Insulin |
| Needle Wall Type | Thin Wall |
| Syringe Scale | 0.3 mL graduations |
| Sterile | Sterilized product |
| Sterilization Method | Gamma radiation |
| Latex Statement | Not made with natural rubber latex |
| Disposable | Disposable product |
Why shorter is more comfortable?
Most people prefer short needles. They are more comfortable and less threatening, so its easier to start injecting insulin. Short needles also help prevent injections from going into muscle, which can result in pain and poor blood sugar control.
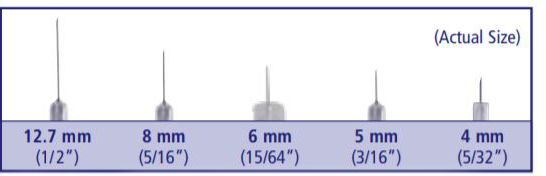
Needle length (mm)
Insulin needles come in different lengths. BD Original Needles are 12.7 mm (1/2″) long, and BD Short Needles are 8 mm (5/16″) long. The BD Nano Needle 4mm (3/16″) and BD Mini Needle 5mm (5/32″) are available only in pen needles. For a comfortable injection experience, shorter needle lengths are recommended and preferred.
Needle gauge (G)
The higher the gauge, the thinner the needle. For example, 32G is thinner than a 31G needle.Insulin needles are available in many gauges (G), or thicknesses. The higher the gauge, the thinner the needle. For example, a 32 G needle is actually thinner and more comfortable than a 29 G needle.
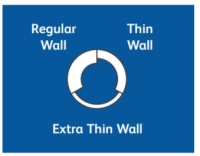
Thin Wall
Thin Wall: As shown in the diagram, the thin wall needle has a narrower steel wall, allowing a greater volume of fluid to pass through it. The flow rate is typically equivalent to that of a needle one gauge larger. This is especially important with very thin needles.
Permanently Attached Needle
Most commonly found in insulin and ‘tuberculin’ syringes. Permanently attached needles, also known as integral needles, reduce the amount of medication waste and allow accurate mixing of different medications into one syringe.
Regulatory Compliance and Quality System
BD Products comply with the regulatory requirements of the region in which these are sold and manufactured.
Sterility
All products which are labeled as #sterile# and released for sale by BD are certified to be sterile per EN 556-1 Sterilization of Medical Devices as long as the package is unopened and undamaged. This product is sterilized via Cobalt 60 – Irradiation. Sterilization cycle development/validation is performed to 10-6 SAL in accordance with current ISO 11137 guidelines.
Biocompatability
This product has been evaluated in accordance with ISO 10993 “Biological Evaluation of Medical Devices”, and complies with all relevant sections.
Pyrogenicity
All products which are labeled as non-pyrogenic and released for sale by BD have been tested per United States Pharmacopeia (USP) chapter (85)- Bacterial Endotoxins Test and meets limits as specified in chapter 161- Transfusion and Infusion Assemblies and Similar Medical Devices.
Quality Control Testing and Release
Representative production samples are collected and inspected in accordance with current applicable product specifications. Inspection records are reviewed and signed off by qualified personnel for product release. The released devices meet applicable BD product specification(s).
Device Characteristics
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437) | No |
| Device labeled as “Not made with natural rubber latex” | No |
| For Single-Use | Yes |
| Prescription Use (Rx) | No |
| Over the Counter (OTC) | No |
| Kit | No |
| Combination Product | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P) | No |
Proper Injection Technique
Proper injection technique is essential to improve consistency in medication delivery and optimize glycemic control. It includes factors such as:
- knowing where the common injection sites are
- understanding how to rotate sites
- ensuring consistent delivery into subcutaneous (fat) layer
- choosing proper needle length
- avoiding injecting into muscle
Why is Proper Disposal Important?
Proper syringe disposal will help to:
- Store and safely dispose of used syringes and lancets
- Protect trash collectors from accidental needlesticks
- Prevent your used syringes from falling into the wrong hands
- Protect the environment.
Product Packaging Information
| Packaging Level | Shelfpack | Case | Each |
| Quantity | 100 | 500 | 1 |
| Length | 17.145 cm | 43.82 cm | |
| Width | 13.97 cm | 18.42 cm | |
| Height | 8.415 cm | 15.24 cm | |
| Weight | 310.0 g | 1.73 kg | 3.636 g |
Shop with confidence
Customer Reviews
You must be logged in to post a review.
Related products
Greiner Bio-One 456085 Venous Blood Collection Tube Plain 13 X 100 mm 6 mL White / Black
Out of stock
BD 305210 Oral Medication Syringe 3 mL Bulk Pack Luer Slip 500/CS
In stock
















Reviews
Clear filtersThere are no reviews yet.