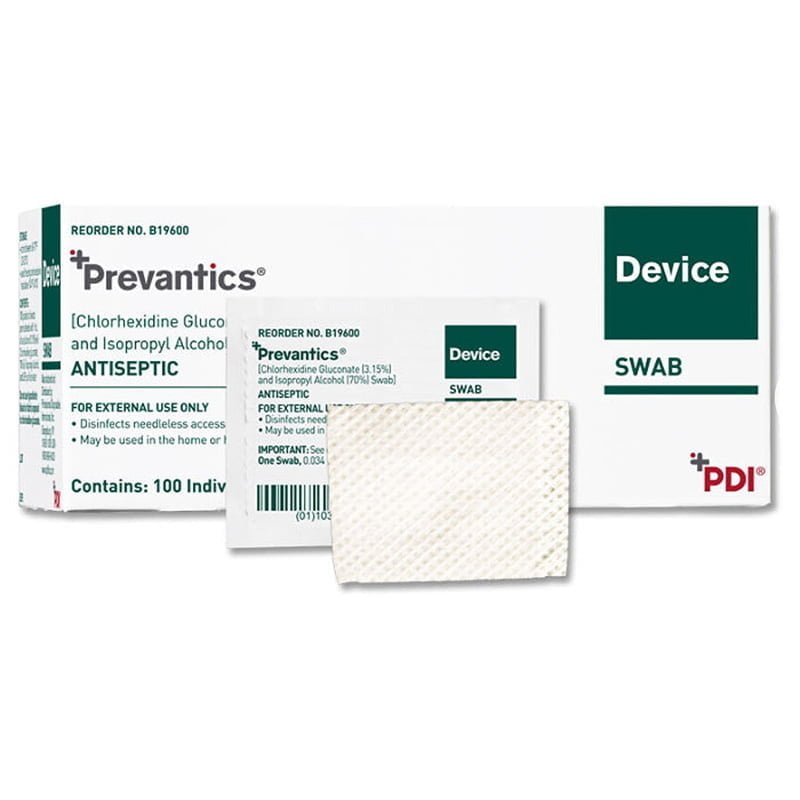
Prevantics® Device Swab & Strip B19600 10 BX/C
32
People watching this product now!
Payment Methods:

Description
Scrub and Dry Time
- 5 second scrub time followed by 5 second dry time
Formulation
- 3.15% (w/v) chlorhexidine gluconate (CHG) and 70% (v/v) isopropyl alcohol (IPA)
Applicator
- Intuitive, easy-to-use prep pad format provides friction to remove microorganisms from grooves and threads of connectors
Efficacy
- Achieved a >4.0 log10 reduction versus a leading alcohol impregnated cap1 against 7 tested clinically relevant microorganisms known to cause CLABSIs2
Guideline Recommended
- Effectively scrub the hub prior to and between each line access, as recommended in the Infusion Nurse Society (INS) Guidelines
FDA Cleared
- First and only 3.15% CHG/70% IPA swab to receive 510(k) clearance from the US Food and Drug Administration (FDA) for disinfecting needleless access sites prior to use
Convenient Format
- Strip format hangs conveniently on an IV pole for point-of-care accessibility
Use Sites
- Disinfecting needleless access sites (needleless connectors, injection ports, and access ports) and tops of blood culture collection bottles.
Independent Data
- Over 15 independent clinical studies demonstrating clinical efficacy of Prevantics products
Compliance
- US Centers for Disease Control and Prevention
- Infusion Nurses Society
- Society for Healthcare Epidemiology of America
- The Joint Commission National Patient Safety Goals
- Infectious Diseases Society of America
- Association for Professionals in Infection Control and Epidemiology
- Association for Vascular Access
Shop with confidence
Terms And Conditions |
Refund Policy
×
Customer Reviews
Rated 0 out of 5
0 reviews
Rated 5 out of 5
0
Rated 4 out of 5
0
Rated 3 out of 5
0
Rated 2 out of 5
0
Rated 1 out of 5
0
Be the first to review “Prevantics® Device Swab & Strip B19600 10 BX/C” Cancel reply
You must be logged in to post a review.
Related products
DYNAREX 1303 TOWELETTE INDIVIDUAL PK ANTISEP BZK, SCENTED CS1000
Rated 0 out of 5
In stock
$5.99 – $36.99Price range: $5.99 through $36.99
Select options
This product has multiple variants. The options may be chosen on the product page
SKU:
1303



















Reviews
Clear filtersThere are no reviews yet.