Frequently Asked Questions About the Norovirus Vaccine

Norovirus Vaccine:
Frequently Asked Questions
As winter approaches, seasonal viral infections start spreading at a faster pace. Many of these infectious agents are foodborne like the Norovirus infection which affects the gastrointestinal tract. According to the statistics reported by the journal of Infectious Disease Clinics of North America, in the United States, the disease has an endemic status as it causes 19 to 21 million illnesses every year. As a preventive measure, the development and administration of a Norovirus vaccine is the only solution. But how close are we to developing a Norovirus vaccine and do we have potential vaccines in the pipeline?
Developing a Norovirus vaccine is the primary goal in health and care research
In this article, let us learn about Norovirus, and its symptoms, as well as answer some of the frequently asked questions regarding the Norovirus vaccine.
What is Norovirus?
Norovirus is a contagious virus that gets transmitted from one person to another. It is known to cause severe gastroenteritis or swelling of the stomach and intestine. The condition is characterized by nausea, vomiting, and diarrhea.
Several different strains of Norovirus have been identified which include viruses belonging to family Caliciviridae. The virus most commonly associated with the infection is Norovirus GII.4. Being a foodborne virus, the infection is also transferred through contaminated food. If you have been infected, it takes about 12 to 24 hours for the symptoms to appear.
“Every 1 in 5 acute cases of gastroenteritis worldwide are caused by Norovirus.”
Centers for Disease Control and Prevention (CDC)
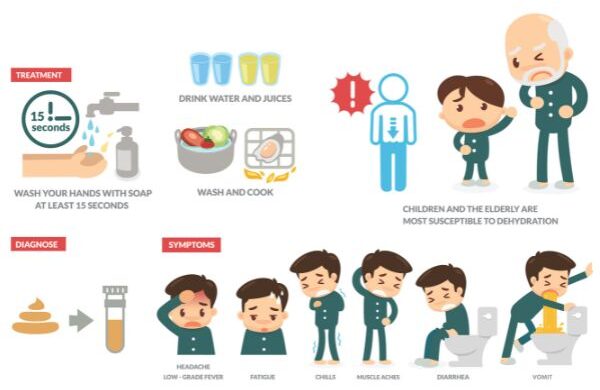
Symptoms of Norovirus Infection
The following symptoms have been associated with Norovirus infection:
- Nausea
- Vomiting
- Diarrhea
- Pain in the stomach
- Headache
- Fever
- Body pain
Symptoms and preventive strategies for Norovirus infection
Norovirus Vaccine Development: Where Are We?
Since the first Norovirus outbreak in 1968, scientists have been working on vaccine development. Still, for now, there are no licensed Norovirus vaccines but some of the options are in the pipeline. These include the following vaccines with respect to the strains against which they have efficacy as per the data published by the World Health Organization (WHO):
| Norovirus Strain | Vaccine Options | Clinical Trials Phase | Route of Administration | Type of Vaccine |
| Bivalent GI.1 and GII.4 | Hillevax
|
Phase III | Intramuscular | Subunit, recombinant, polysaccharide, and conjugate vaccine |
| Chinese vaccine developed by the National Vaccine and Serum Institute | Phase II | Intramuscular | Subunit, recombinant, polysaccharide, and conjugate vaccine | |
| Quadrivalent GI.1, GII.3, GII.4, and GII.17 | Anhui Zhifei Longco Biologic Pharmacy Co. Ltd. | Phase II | Intramuscular | Subunit, recombinant, polysaccharide, and conjugate vaccine |
| Monovalent GI.1 or GII.4, or bivalent GI.1 and GII.4 | Vaxart | Phase II | Oral | Viral vector vaccine |
Norovirus Vaccine: Frequently Asked Questions
With the development and testing of the new investigational mRNA-1403 multivalent Norovirus vaccine, many queries have started puzzling the minds of the people who live in the regions where the infection keeps coming back every now and then.
The UK government as well as the pharmaceutical company Moderna have issued statements about the results obtained by phase I and II clinical trials. The following section covers some of the frequently asked questions about the new Norovirus vaccine regarding its efficacy, future prospects, and plans:
1. Is there a Norovirus vaccine on the market?
At present, no. There is no licensed Norovirus vaccine. However, an mRNA vaccine has recently given positive results during phase II clinical trials. According to Dr. Patrick Moore, the chief investigator of this trial conducted in the UK, this investigational mRNA Norovirus vaccine will soon go into phase III clinical trial called Nova 301 trial which will then continue for 2 years in countries including Japan, Australia, and Canada.
2. How does this mRNA-1403 investigational Norovirus vaccine work?
This mRNA (messenger ribonucleic acid) vaccine contains a single strand of a messenger RNA molecule which is introduced into the human cell. Our cells read the instructions written on this mRNA chain and make proteins needed for the identification of Norovirus. This way, our immune system becomes prepared to fight the virus by preparing antibodies.
3. When are the trials expected to begin?
The company plans to start the clinical trials in the UK by October 2025. For this purpose, at least 25,000 participants are to be recruited from 27 NHS primary and secondary care sites. Among these, 20,000 will be elderly while 5,000 participants will be between the ages of 18 to 59.
The trial will include people resigning within the UK as well as those in other countries like the USA, Australia, Japan, etc.
4. What are the inclusion criteria for the participants?
Like most other clinical trials, this phase III process will also recruit individuals who are 18 or above in age. However, the scientists are keener to test the new Norovirus vaccine on the elderly who are 60 or above. This is because such individuals are at a higher risk of getting infected with Norovirus infection.

Norovirus-induced gastroenteritis causes severe stomach pain like other infectious diseases
5. How will the phase III clinical trials for the new mRNA-based investigational Norovirus vaccine take place?
The participants will be divided into two groups. One will receive an intravenous (IV) jab of the new mRNA-based investigational Norovirus vaccine while the participants of the second group will be given a jab of saline.
As the trial will be observer-blinded as well as placebo-controlled, none of the participants from both groups will know whether they have been given the Norovirus vaccine or saline.
6. What is expected from the Norovirus vaccine Nova 301 trial?
Phase III clinical trials are being conducted to evaluate the extent of the immune response produced by the vaccine. In addition, it is also crucial to find out for how long the Norovirus jab will provide protection against the winter vomiting bug infection.
7. Which company will have the rights to the new vaccine?
The development of this investigational Norovirus vaccine is a joint effort of the well-known pharmaceutical company Moderna, the National Institute for Health and Care Research (NIHR), and the Department of Health and Social Care (DHSC) which comes under the UK Health Security Agency (UKHSA).
8. What will be the next step once the phase III trials are successful?
According to the scientists working in Moderna, if the vaccine shows an efficacy of 65% or higher in phase III clinical trials, it can be considered a clinical success. In this case, the next step would be to submit a marketing application to the regulators by the end of 2026.
After a year-long review period followed by affirmative results, the vaccine will be tested in different age groups including children and teenagers. If everything goes well, this vaccine will be the first mRNA vaccine for Norovirus.
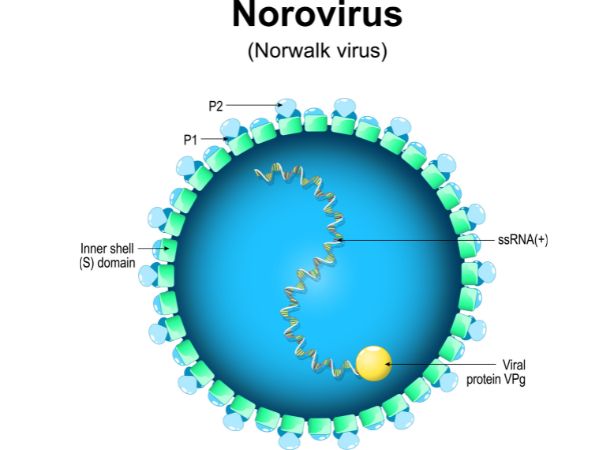
Structure of Norovirus or winter vomiting bug
Preventive Measures for Norovirus Infection and Other Infectious Diseases
Until the approval of the new Norovirus vaccine, our best chance is to adopt preventive measures against the Norovirus infection so that the risk of getting sick can be lessened. For this purpose, the following measures should be kept in mind:
Keep Your Hands Clean
As Norovirus is highly contagious, it is important to minimize the risk of exposure. Wash your hands with good-quality soap and clean water. Rinse the soap for at least 20 seconds before washing. If soap and water are not available, you can also use hand sanitizers which are alcohol-based. Hand hygiene is essential especially after using the toilet, before or after having a meal, as well as after changing a diaper.
Focus on Disinfection
It is also recommended to properly clean and disinfect any contaminated surfaces. If the surfaces are porous, mix one and two-thirds cups of chlorine bleach in one gallon of water and wipe them thoroughly. In the case of non-porous surfaces, one-third cup of chlorine bleach can be added to one gallon of water.
Keep Your Clothes Clean
If you are already sick with the infection, make sure to wash your clothes thoroughly as they might have vomiting or some traces of stool on them. For this purpose, use a good-quality detergent and hot water. You can also wash them in a washing machine on the highest possible heat.
Avoid Raw Seafood
As Norovirus is a foodborne infection, wash your seafood thoroughly to avoid the risk of infection. Special focus should be given to shellfish, oysters, and clams. Moreover, it should be made sure that the food does not remain raw and is evenly cooked before consumption.
Clean Fruits and Vegetables
Make it a habit of washing the fruits and vegetables thoroughly before eating them.
Quarantine Yourself
If you have been in contact with someone who has Norovirus infection or you have some of the symptoms of the infection, it is best to quarantine yourself and stay away from other people to reduce the risk of person-to-person transmission.
When your symptoms disappear, wait for another 48 hours before socializing.
Only Travel When Necessary
During the outbreaks of Norovirus infection, it is best to stay indoors as much as possible. However, if traveling is necessary, make sure to take all the necessary measures. Wear a mask, use hand sanitizer frequently, and avoid drinking or eating from street vendors.
Getting vaccinated for Norovirus can prevent highly contagious disease
The above-mentioned products, along with many other medical supplies , can be ordered from Health Supply 770, a reliable name when it comes to medical products. They have a 30-day money-back guarantee and provide your products to you in the shortest possible time. Click the link given in the article to check out the wide range of instruments and devices for the diagnosis and management of infectious diseases.
Bottom Line
Norovirus infections have been on the rise for the past few decades. Every winter, the cases related to the disease elevate and put extreme pressure on healthcare as most people require proper medical treatment to get well.
The infection is associated with severe gastroenteritis which causes vomiting, diarrhea, as well as abdominal pain. Therefore, considering the importance of infection prevention, efforts have been made to develop a Norovirus vaccine that is effective against the disease.
The new Moderna vaccine, called mRNA-1403 Norovirus vaccine, has shown promising results in the phase I and II clinical trials. Although it is yet to start its phase III trials, there are hopes for positive results as this Norovirus vaccine is expected to be proven safe and effective. But for now, our only chance is to do everything in our power and capacity to avoid getting infected.
For purchasing top-quality medical products, reliable vendors like Health Supply 770 should be approached. They ensure the provision of quality products along with satisfactory services.
References
https://www.who.int/teams/immunization-vaccines-and-biologicals/diseases/norovirus