How do you use Biological Indicators for Sterilization?
While dealing with hospitalized patients, it is significant to minimize the chances of transmission of pathogenic entities by any means which may act as the causative agents of various infections in them. Therefore, important steps are to be taken in order to achieve the maximum sterilized environment around diseased subjects. One of these practices is the employment of a sterilization process on the medical apparatus and devices used to take samples, conduct operations, administer medications, etc.
But, as the sterilization is conducted, another necessity emerges regarding the level of sterilization which is achieved after the process has been completed. To ensure that the extent of sterility achieved is exceeding the requirements or is at least equal to it, indicators are employed which include chemical as well as biological indicators. In this article, a detailed analysis of these biological indicators has been presented along with their method of utilization.
An Introduction to biological indicators
Biological indicators (BIs), or as they are frequently called, the living indicators are the indicating agents with high sensitivity to the environmental fluctuations which are employed to ensure the sterilization processes.
Composition of the biological indicators
The composition of the biological indicators is based on the varying kinds of spores of different bacterial strains which include:
- Bacillus subtilis
- Bacillus atrophaeus
- Geobacillus stearothermophilus
These spores are collected and manufactured in the form of strips called ‘spore strips’. These strips containing bacterial spores attached to a paper strip come in glassine envelopes or are packed into vials that are to be opened just prior to their employment. The spore count of one such strip exceeds a million.
How is a biological indicator used?
The biological indicators are as simple in their activity as they are in their composition. The following steps are the guidelines for the employment process of BIs:
- Choose the sterilizer in which the sterilization is to be conducted. Make sure that the sterilizer is fully loaded.
- Biological indicator spore strips are placed into this loaded sterilizer and the sterilization process is started.
- After the sterilization process is completed, collect the biological indicator and mix it well with an incubation media in a glass vial or ampoule.
- Check the manufacturer’s pre-set specifications for the temperature at which the collected biological indicator plus its media are to be incubated.
- Put the mixture into the incubator for the pre-determined time duration which is generally based on 12, 18, 24, and 48 hours.
- Once this time period comes to an end, collect the biological indicator and interpret the results based on the color change or color retention of the media.
How to interpret the results using biological indicators?
With the use of biological indicator spore strips, the outcome of the test can be one of these two:
- Positive BI: If, after the completion of the sterilization as well as incubation process, no color change is observed in the spore strips, it is manifested that the sterilization process is in fact not working according to the requirements. It is understood that the efficiency of the method is lacking and further adjustments are needed to fix the errors.
- Negative BI: If the color of the incubation media is changed after the sterilization process, it is a clear indication of the fact that the process of sterilization is up to the mark and no further adjustments are needed. This happens when the spores contained within the spore strips are completely killed during the process.
Generally, it is recommended to have a positive control while performing the experiments of the sterilization process so that the efficacy of the biological indicator can be checked. This positive control must be selected from the same batch to which the test indicator belongs.
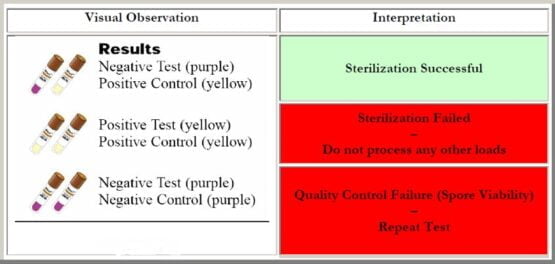
Visual observations of the biological indicator with respect to the control as well as the result interpretation
What should be done to achieve negative results?
If positive results have been obtained from a sterilization process, it is important to locate the point of the problem. In most cases, the issue with the sterilization process is due to one of the following reasons:
- The issue of insufficient time is likely to happen which needs to be increased in order to achieve complete sterilization. This problem can be fixed by adjusting the time gauge or by readjustment of the cycle parameters, etc.
- In some cases, the sterilizer is not in the right condition to give the required results. The issues may include the clogging of the vent liner as well as the filters, faults in the vacuum pumps, maladjustment of the control valves, lesser come-up time, low pressure of the steam, or inappropriate gasket seal of the door, among others.
- There is a high possibility of human errors which include an improper choice of packaging material, putting the material in bulk, poor loading technique, inaccurate usage method used for the sterilizer, etc.
Conclusion
Biological or living indicators are the easiest as well as one of the most reliable ways when it comes to the indication of the effectiveness of a process of sterilization. The spore strips, based on the bacterial spores, are not only highly economical but also have the ability to give rapid results.
Moreover, the interpretation of the results is uncomplicated and needs no special training to comprehend. Therefore, it is not wrong to say that the use of biological indicators not only does the job very efficiently but also saves a lot of time and effort.