BD 309680 Tray Convenience Bulk STE (Pack of 120)
$129.88
221 in stock
221 in stock
Payment Methods:

Description
BD 309680 – TRAY CONVENIENCE BULK STE
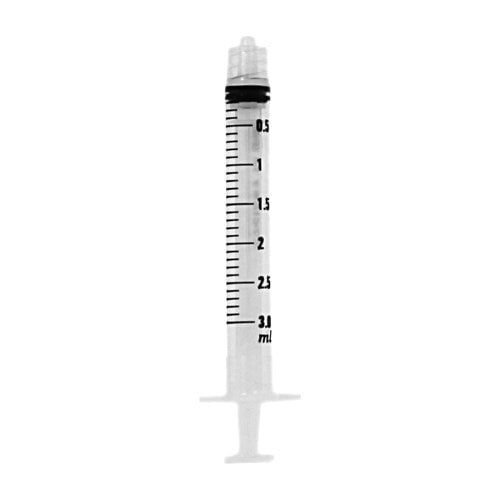
60 mL BD Luer-Lok Syringe Bulk Sterile Pharmacy Convenience Tray
BD sterile syringe convenience trays help pharmacy staff save time and effort when batch-preparing syringes.
| CE Mark | Product is CE-marked |
| Pyrogen Free | Product is pyrogen free |
| Syringe Tip Orientation | Concentric |
| Syringe Tip Type | BD Luer-Lok |
| Syringe Scale | 1 mL graduations |
| Sterility | Sterile |
| Total Shelf Life | 1825 |
| Volumetric Accuracy | +/- 5% *for 1ml or smaller,accuracy below 0.2ml is+/- 0.07ml |
| Sterilization Method | Radiation |
| Latex Free | Not made with natural rubber latex |
BD Luer-Lok Syringe
Bring security to critical applications
The BD Luer-Lok syringe is ideal for critical clinical applications that crucially require you to avoid potential needle disengagement, medication leakage, spray or tubing disconnect.
BD Luer-Lok thread
The BD Luer-Lok thread provides a secure connection.
Plunger rod design
Each disposable syringe features a tapered plunger rod for ease of aspiration and a positive plunger rod stop.
Ease of use
BD syringes feature a clear barrel with bold scale markings.
Graduation
The 5-mL syringe’s scale has 1/5 mL graduation.
BD Luer-Lok Tip
BD Luer-Lok tip is generally used for injections requiring a secure connection of the syringe to another device.
Regulatory Compliance and Quality System
BD Products comply with the regulatory requirements of the region in which these are sold and manufactured.
Sterility
All products which are labeled as #sterile# and released for sale by BD are certified to be sterile as long as the package is unopened and undamaged. For those products labeled #sterile fluid path#, only the fluid path is sterile. This product is primarily sterilized via E-beam. Sterilization cycle development/validation is performed to 10-6SAL in accordance with current ISO 11137 guidelines.
Biocompatability
This product has been evaluated in accordance with ISO 10993 “Biological Evaluation of Medical Devices”, and complies with all relevant sections.
Pyrogenicity
All products which are labeled as non-pyrogenic and released for sale by BD have been tested per United States Pharmacopeia (USP) chapter 85 – Bacterial Endotoxins Test and meets limits as specified in chapter 161- Transfusion and Infusion Assemblies and Similar Medical Devices.
Quality Control Testing and Release
Representative production samples are collected and inspected in accordance with current applicable product specifications. Inspection records are reviewed and signed off by qualified personnel for product release. The released devices meet applicable BD product specifications.
Device Characteristics
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437) | No |
| Device labeled as “Not made with natural rubber latex” | No |
| For Single-Use | Yes |
| Prescription Use (Rx) | Yes |
| Over the Counter (OTC) | No |
| Kit | No |
| Combination Product | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P) | No |
Shop with confidence
Customer Reviews
You must be logged in to post a review.
Related products
BD 329515 Insulin Pen Needle AutoShield Duo 30 Gauge 5 mm Length Automatic Locking Shield 800/CS
In stock
BD 930825 Prep Solution Chloraprep Scrub Teal 26 Ml Applicator 2% / 70% Strength Chg Isopropyl Alcohol Sterile 25/CS
In stock
Greiner Bio-One 24121 Blood Collection Set 23 Gauge 3/4 Inch Needle Length Safety
Out of stock

















Reviews
Clear filtersThere are no reviews yet.