FAQs about iHealth COVID Tests: Everything You Need to Know
As per recently collected data from healthcare setups, it seems that COVID is not yet ready to go. Every time a new one appears, a prominent spike can be seen in the number of coronavirus cases. The situation thus emphasizes the importance of taking a rapid COVID test as soon as either the symptoms start appearing or after a recent encounter with a patient. In this regard, multiple kinds of rapid tests are available, one of which is the iHealth COVID test.
But what exactly are these iHealth COVID tests and how do they work? Let us answer some of the most frequently asked questions about this test in the following article.

What is the iHealth COVID Test?
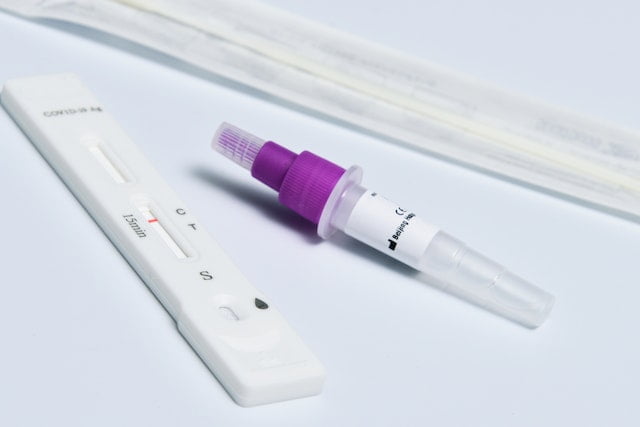
The iHealth COVID rapid test is an antigen-based lateral immunoassay method employed for the identification of the nucleocapsid of the SARS-CoV-2 virus in an individual. This non-invasive test is non-prescription and can be easily taken at home without any professional help.
Who can take the iHealth Rapid COVID Test?
The test is suitable for individuals aged 2 years or older. For children, the sample can either be taken by a parent or a guardian as well as by a healthcare professional in the presence of a parent or a guardian.
However, for adults, the test can be performed either by the patient herself/himself or by a healthcare provider. People can also approach telehealth facilities to clear any doubts while doing an at-home test.
Guidelines Regarding the Frequency of Testing
According to guidelines issued by the Food and Drug Administration (FDA), for the confirmation of an active COVID-19 infection in a symptomatic patient, the test should be repeated twice within three days with a gap of 48 hours between the two.
Moreover, for individuals who are asymptomatic but suspect the presence of SARS-CoV-2 virus in their body, it is advised to take the test three times within 5 days with a 48-hour interval between each.
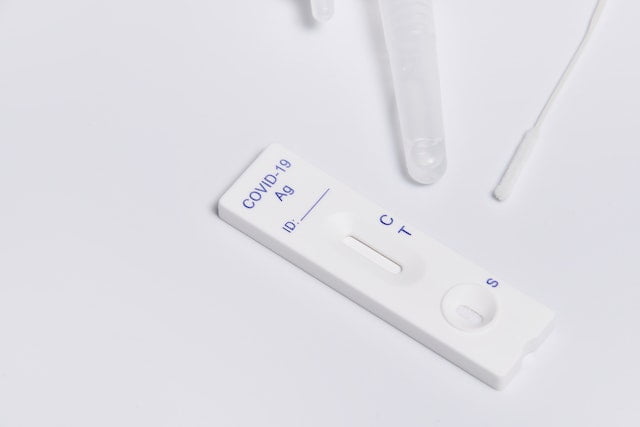
How to Use the Test? A Step-by-Step Guide
An iHealth COVID test is conducted just like other rapid tests. Its step-by-step procedure has been detailed by the FDA and follows the given procedure:
Sample collection:
The iHealth rapid antigen test is conducted using a sample taken from the anterior portion of an individual’s nasal cavity using a nasal swab.
Antigen-antibody reaction:
After collection, this swab is transferred into a prefilled tube that has sealed solutions in it. This solution interacts with the viruses present in the nasal sample. As a result, viral antigens are exposed to the antibodies and a reaction occurs.
Indication of results:
This solution is then poured into the sample port of the COVID-19 test card present in the kit and waits for 15 minutes. As a result of this qualitative detection, a positive or negative result is indicated which can be interpreted based on the following table:
| Status on 1st Day of Testing | 1st Result Day | 2nd Result Day | 3rd Result Day | Interpretation |
| With Symptoms | Positive | N/A | N/A | Positive for COVID-19 |
| Negative | Positive | N/A | Positive for COVID-19 | |
| Negative | Negative | N/A | Negative for COVID-19 | |
| Without Symptoms | Positive | N/A | N/A | Positive for COVID-19 |
| Negative | Positive | N/A | Positive for COVID-19 | |
| Negative | Negative | Positive | Positive for COVID-19 | |
| Negative | Negative | Negative | Negative for COVID-19 |
Addressing Common Questions about Accuracy, Reliability, and Shelf-life
Antigen tests are always less accurate than molecular tests but this fact does not deny their importance when it comes to self-testing and at-home detection of the infection. The accuracy, reliability, cost, as well as expiry of the iHealth rapid test have been addressed below:
Accuracy:
Like other antigen tests, the iHealth rapid antigen test is also highly accurate with a percentage of 94.3% for lay users. Despite this high value, repeated testing is recommended to eliminate the chances of an inaccurate result.
Reliability:
The test has also been found to have correctly identified 98.1% of the negative samples, a number which indicates its reliability.
Expiry:
The iHealth COVID test kits have an expiry of 12 to 15 months.
Cost:
An iHealth rapid test kit costs $17.98 with free shipping. This pack contains materials to conduct two tests.
Differences between iHealth and other COVID Tests
iHealth rapid antigen COVID test kits are superior in performance to many other COVID tests owing to their ease of conduction.
Moreover, the results obtained from this test are clearer and easier to interpret as compared to others which not only take longer time to indicate the results but are also difficult to understand.
Furthermore, the iHealth test kits have minimum components in them which renders the testing process quick and uncomplicated for the user.
Proper Storage and Handling of Test Kits
For the storage of iHealth rapid test kits, a temperature of 36 to 38°F (2 to 30°C) is recommended by the FDA.
However, at the time of use, the test kits should be kept at room temperature which should not exceed the range of 65 to 86°F (18 to 30°C).
Once the expiry date of the test kits has passed, these should be immediately discarded.

The iHealth COVID test kits mentioned in this article, along with many other medical supplies, can be purchased from Health Supply 770, a reliable name when it comes to medical products. They have a 30-day money-back guarantee and provide your products to you in the shortest possible time.
Conclusion
iHealth COVID tests are one of the most reliable test kits when it comes to the detection of SARS-CoV-2. Being a non-prescription test, anyone in need can access it to conduct it at home with or without the help of a healthcare provider.
The accuracy of the test indicates that it gives reliable results in most of the cases with a narrow margin of error. In the present year, the FDA has also increased the expiration date of iHealth COVID test kits up to 15 months.
Overall, the iHealth rapid test kits are reliable, accurate in performance, and highly affordable. Make sure to grab some of these test kits to have stock at home.
For making a purchase, only check reliable suppliers like Health Supply 770 to get top-quality products.
References: