Automated External Defibrillator (AED): A Shortage Costing Lives
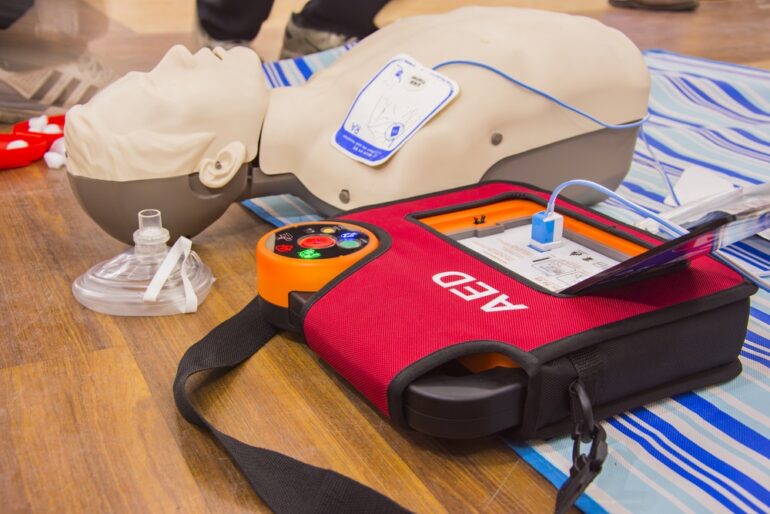
The COVID-19 pandemic, undeniably, has impacted the world in multiple ways. From the unprecedented medical burden to the still ongoing economical instabilities, the pandemic has affected every dimension of human needs and wants. It appears that neither any service nor any commodity has remained untouched. However, some affected articles have a far major influence over others. The supply chain issues regarding the frequently employed medical devices have rendered a shortage of life-saving products in markets all over the world. One such product which has been listed as “short in supply” by the FDA is the automated external defibrillator. Let us first understand what an AED is before looking into the causes of its shortage.
What is an AED?
An automated external defibrillator, abbreviated as AED, is an electrical device employed to make a diagnosis of life-threatening cardiac issues. It is a portable defibrillating system that works by detecting the abnormalities in the cardiac periodicity and defibrillating i.e. aiding the heart to retrieve its normal rhythm by the use of electric current.

An AED portable system
When is an AED needed?
An AED is useful if a person experiences any of the following conditions:
- Cardiac arrhythmiase. too high or too low cardiac rhythms. The patient may experience palpitation, difficulty in breathing, pain in the chest, or lightheadedness followed by fainting.
- Ventricular fibrillation which is indicative of the uncoordinated electrical activity within the heart resulting in the development of abnormal cardiac rhythms.
- Pulseless ventricular tachycardia which is manifested by an elevated heart rate due to the rapid movement of ventricles. In this state, the patient becomes unconscious and has no pulse.
Manufacturers and suppliers of AED
The following are the suppliers of the automated external defibrillator:
- Avive Solutions, Inc.
- Mindray
- Metrax GmbH
- ZOLL Medical
- Cardiac Science Corporation
- Medtronic
- Nihon Kohden
- Philips
- Defibtech, LLC
Why a shortage does exist?
The Food and Drug Administration or the FDA has enlisted AED in the “device shortage list” reasoning that some of the essential parts, semiconductors to be precise, required to manufacture the device are stuck in the line due to supply issues. Moreover, higher demand for the AED device has also disturbed the manufacturing and consumption balance thus shifting it more towards the latter.

AED listed in the device shortage list by the FDA
When will the supply be resumed normal?
Although the FDA indicated that the supply shortage for the parts of many medical devices, including the AED, is likely to be resolved by the end of 2022, it seems like it will still take some time to resume to normal as the demand and manufacturing gap is to be fulfilled by the companies.
Are you at risk?
If you are a heart patient or included in a high-risk group, having an AED with you is a good choice. However, in times of shortage, some of the alternative devices are still easily available. The most significant one of these includes an implantable cardioverter-defibrillator (ICD) which is a battery-operated system to be implanted into the body. Lead wires connect the device to your heart which is also responsible for the transmission of electric current to the organ when needed.
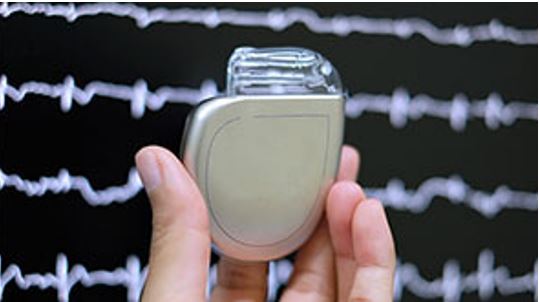
Implantable cardioverter-defibrillator (ICD)
The latest version of ICD is the less invasive subcutaneous-implantable cardioverter defibrillator (S-ICD) which is implanted subcutaneously i.e. under your skin to perform a similar function as expected from an AED or ICD. The difference is that S-ICD does not have a direct connection with the heart. Rather, it is placed under the armpit and is led to the exterior side of the heart via an electrode.
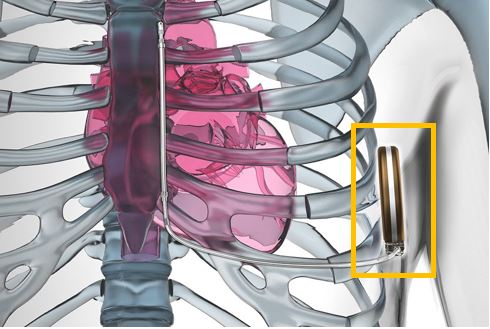
Subcutaneous-implantable cardioverter defibrillator (S-ICD)
Cost comparison between an AED and ICD
When it comes to cost, both the automated external defibrillator as well as the implantable cardioverter defibrillator remain nearly equal. An AED costs somewhere between 1400 to 2200 USD. In comparison, an ICD is being sold at approximately 2500 USD indicating that there isn’t a wider difference in the prices of both kinds of defibrillators. Similarly, their efficacy can be compared which, like their prices, is satisfactorily identical enough thus rendering them suitable to be used as a replacement for each other.
Conclusion
Shortages in the supply of medical devices can result in life-threatening situations in the worst-case scenario. Reasons behind these shortages can be the unavailability of certain parts of the device due to reduced supply or hindrances in production. Thanks to the substitutes which are available for almost every device, the time until the supply is resumed again can be covered. However, steps should be taken to minimize the problems regarding the smooth supply of medical equipment even during travel restrictions or import-export bans.